Precision Therapeutics Platform

Discovery Automation
High-Throughput Phage Discovery and Characterization Next-Generation SequencingDiscovery Automation
High-Throughput Phage Discovery and Characterization
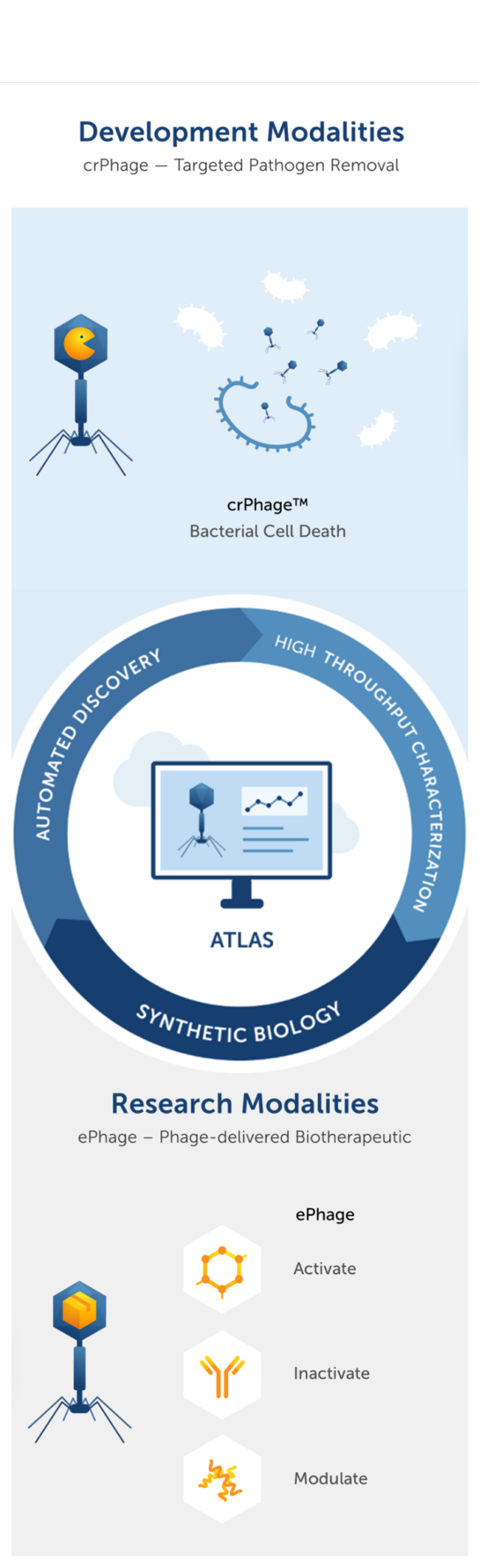
Utilizing high-throughput liquid handlers, Locus is able to rapidly identify and characterize bacteriophage libraries against any bacterial target.
Our team collects natural bacteriophages from the environment and uses a high-throughput robotic platform to isolate and characterize phages with the desired properties. Selected phages are combined into cocktails that can kill the clinically-relevant strains of the target bacterial species. We screen phages against target strains using a proprietary high-throughput system to quickly identify a cocktail with optimized activity across thousands of pathogenic isolates to serve as the starting point for an engineered phage cocktail.
Next-Generation Sequencing
Next-generation sequencing (NGS) technology enables Locus to rapidly capture the genomic sequence of each phage.
Having complete genomic sequences for both the phage products and their bacterial targets allows us to thoroughly understand the genetics of these organisms. Paired with the phenotypic characteristics captured during high-throughput characterization, we can rapidly identify and select bacteriophages for engineering. Moreover, our database containing thousands of bacteriophage genomes and phenotypic characteristics enables us to understand bacteriophage better than anyone else.

Synthetic Biology
CRISPR-Cas3 Engineering
Locus is the world’s leading developer of products based on CRISPR-Cas3 systems.
CRISPR evolved as a bacterial immune system, which protects bacterial cells from invading viruses. Cas3 is a powerful exonuclease that shreds the DNA of targeted bacterial cells beyond repair, leading to rapid and predictable cell death. We use Cas3 to dramatically increase the potency of crPhage compared to their natural phage counterparts where we ensure that every infection event results in the death of the bacterial cells
Beyond CRISPR
In the pursuit of developing crPhage Locus has become the world’s leading experts in engineering bacteriophage.
The very nature of engineering CRISPR-Cas3 into the genomes of bacteriophage has forced us to become intimately familiar with bacteriophage genomics and phenomics. This enables us to manipulate traits that natural bacteriophage may or may not have, therefore allowing us to enhance desirable attributes or diminish unfavorable characteristics. Furthermore, we are leveraging the bacteria-bacteriophage relationship to precisely deliver and manufacture biotherapeutics within the human body.

Atlas
Knowledge Base
Locus has built a platform that captures and integrates all the data generated across our company.
Recognizing the power of multivariable datasets (e.g., structural and functional, diagnostic and demographic), we invested in developing a single source, Atlas, that captures all of the data generated, collected, and purchased by the company. Having a centralized informatics function enables data scientists to generate unbiased insights regardless of business area. The most valuable function Atlas provides us is the ability to leverage captured data to see forward in time and make data-informed predictions.

Predictive Algorithms
Locus uses proprietary informatics algorithms and machine learning to further automate Discovery processes and provide data-informed predictions across the company.
We can develop models that allow us to better understand variables we haven’t measured yet. Combining genomic and functional datasets enable us to understand how certain organisms may interact with one another. Leveraging data generated from early Locus developed drugs enable us to build drugs against more complex organisms or consortia of organisms. The insights provided using predictive algorithms, allows the team to move quicker, make decisions more confidently, and develop novel precision medicines faster.
CRISPR-Cas3 Gene Editing Platform
CRISPR-Cas3 offers a unique approach to human genome editing with the ability to produce long-range deletions.
Our IP portfolio enables the use of Cas3 human gene editing, a capability that we have been incubating since the inception of Locus. The ability to produce long-range deletions makes it possible to inactivate and/or remove of undesirable genetic material (e.g., viral genomes). Cas3 genome editing offers us another unique tool in its pursuit of using precision medicines to revolutionize the treatment of disease.